Beautiful Work Info About What Allows Electrons To Flow Freely
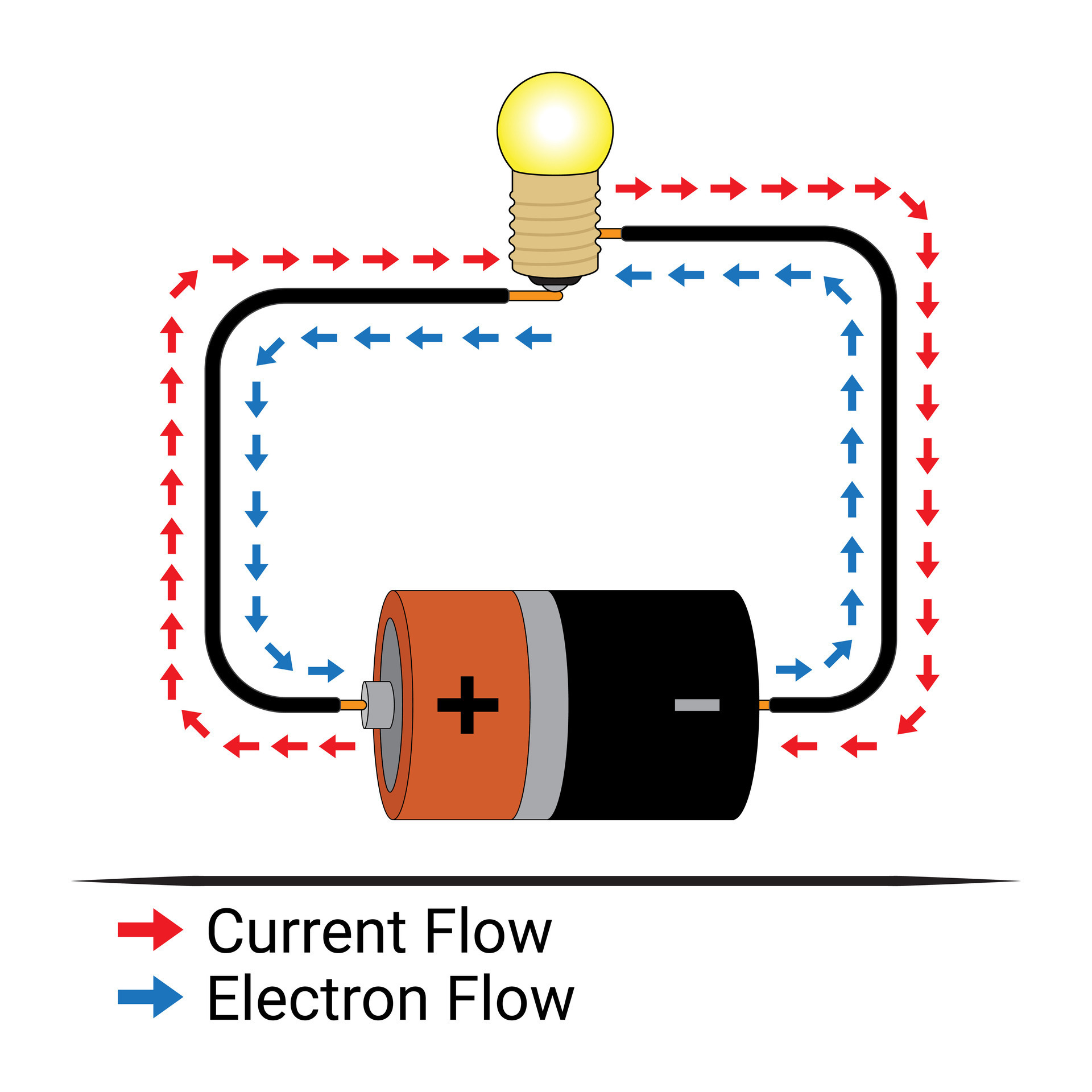
Electron Flow And Current 25747601 Vector Art At Vecteezy
The Great Electron Escape
1. The Electron's Quest for Freedom
Ever wondered what makes electricity possible? I mean, really, think about it. We flip a switch, and BAM! Lights. Toaster works. Phone charges. It's like magic, but it's actually just tiny particles called electrons doing their thing. The real question is, what allows electrons to flow freely and power our world?
Imagine electrons as tiny, energetic kids constantly bouncing around. In some materials, like copper in your wiring, these kids have a pretty easy time moving from one atom to the next. In others, not so much. Think of it like trying to run through a crowded room versus an empty field. That difference? It's all about the material's atomic structure and how tightly it holds onto its electrons.
So, the keyword here is "free electrons." They're the rockstars of the electrical world. These are the electrons that aren't strongly bound to any particular atom. They're like the nomads, roaming the land of the material, just waiting for a chance to hitch a ride on the electrical current express. It's the availability of these free electrons that determines how well a material conducts electricity.
Without these wandering electrons, we'd be stuck in the dark ages. No smartphones, no internet (gasp!), no Netflix binges. So, let's give a little appreciation to those tiny, free-flowing particles that make modern life possible, shall we?
Atomic Structure
2. Peeking Inside the Atom
Alright, let's get a little bit technical (but not too technical, I promise). The ability of electrons to flow freely hinges on the arrangement of atoms within a material. Think of atoms like tiny solar systems, with a nucleus at the center (the sun) and electrons orbiting around it (the planets). Some atoms hold their outer electrons very tightly, while others are more...lax.
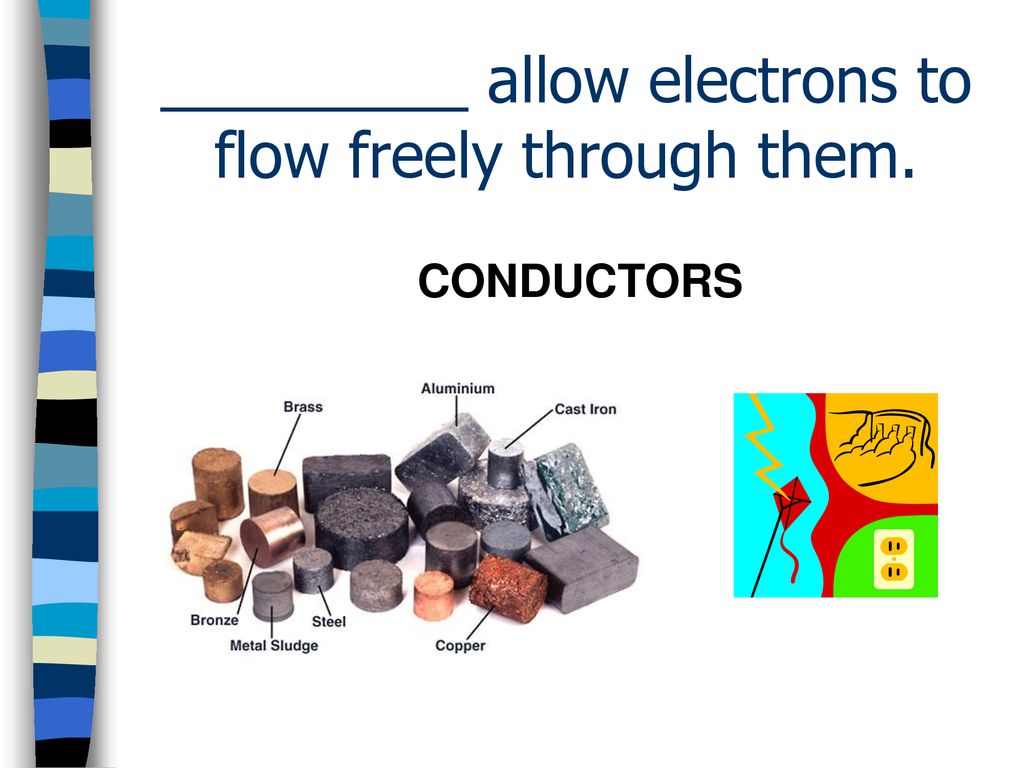
Materials that readily allow electrons to flow, like metals, have a unique atomic structure. Their outer electrons are loosely bound, almost like they're sharing custody with all the neighboring atoms. This creates a "sea" of electrons that can move relatively easily throughout the material. This is why metals are excellent conductors of electricity.
The opposite is true for insulators, like rubber or plastic. These materials have atoms that hold onto their outer electrons with an iron grip. It's like they're super possessive parents who never let their kids out of the house. This makes it very difficult for electrons to flow, which is why insulators are used to prevent electrical shocks and keep electricity where it's supposed to be.
So, the arrangement and binding strength of electrons within the atoms of a material dictates whether electricity can flow. Think of it as atomic-level crowd control, directing the electron traffic for our electrical needs.

The Role of Conductors, Insulators, and Semiconductors
3. A Trio of Electrical Players
In the electrifying world of electron flow, we have three main players: conductors, insulators, and semiconductors. Conductors, like copper and silver, are the superstars. They have a high concentration of free electrons, allowing electricity to flow through them with ease. They're the go-to materials for wiring, power cords, and anything else that needs to carry an electrical current.
Insulators, on the other hand, are the party poopers. They resist the flow of electrons, preventing electricity from going where it shouldn't. Rubber, plastic, and glass are common examples. They're essential for safety, preventing shocks and keeping electrical circuits contained.
Then we have semiconductors, the chameleons of the electrical world. These materials, like silicon, can act as both conductors and insulators, depending on the conditions. This unique property makes them the backbone of modern electronics, from transistors in computers to solar panels.
Semiconductors are kind of like that friend who's always on the fence. They can be convinced to go either way, making them incredibly versatile for controlling electron flow and creating complex electronic circuits. Theyre a crucial element in how all our tech functions.

Electricity Lights Up Your Life!. Ppt Download
External Factors Affecting Electron Flow
4. Temperature, Impurities, and More
Okay, so we've covered the basics of what allows electrons to flow freely. But did you know that external factors can also influence electron movement? Temperature, for instance, can play a significant role. As the temperature of a conductor increases, the atoms within it vibrate more vigorously. This makes it harder for electrons to navigate the material, effectively increasing resistance and hindering their flow.
Impurities in a material can also affect electron flow. Think of it like trying to drive on a road filled with potholes. These impurities disrupt the smooth movement of electrons, scattering them and reducing conductivity. That's why highly purified materials are often used in electronic devices, to ensure efficient electron flow.
Even the physical dimensions of a conductor matter. A thicker wire will allow more electrons to flow compared to a thinner wire, simply because there's more space for them to move. It's like comparing a four-lane highway to a narrow country road.
So, while the material's atomic structure is the primary factor, external conditions can also significantly impact how easily electrons can flow. Its all interconnected, and understanding these influences is key to designing efficient and reliable electrical systems.

Beyond the Basics
5. The Future of Free Electrons
The understanding of what allows electrons to flow freely has revolutionized technology and continues to drive innovation. From the microchips that power our computers to the long-distance transmission of electricity, our ability to control electron flow has shaped the modern world. And the journey doesn't stop here.
Researchers are constantly exploring new materials and technologies to enhance electron flow. Superconductors, for example, are materials that exhibit zero electrical resistance at extremely low temperatures. Imagine electricity flowing with absolutely no energy loss! This could revolutionize power transmission and enable new technologies like levitating trains.
Nanotechnology is also playing a crucial role. By manipulating materials at the atomic level, scientists can create new structures and devices with enhanced electrical properties. This could lead to smaller, faster, and more energy-efficient electronics.
The future of electron flow is bright, with ongoing research paving the way for even more amazing innovations. As we continue to unravel the mysteries of electron behavior, we can expect even more transformative technologies that will shape the world to come. It's truly an exciting time to be alive, powered by the tiny, tireless electrons that flow so freely around us.
+are+free+to+move+from+atom+to+atom.+This+movement+is+called+ELECTRON+FLOW..jpg)
Frequently Asked Questions (FAQs)
6. Common Queries About Electron Flow
Q: What exactly are "free electrons"?
A: Free electrons are electrons that aren't tightly bound to any specific atom within a material. They're able to move relatively freely throughout the material, allowing them to carry an electrical current. They are the key to electrical conductivity.
Q: Why are some materials better conductors than others?
A: It all comes down to their atomic structure. Good conductors have atoms with loosely bound outer electrons, creating a sea of free electrons. Materials with tightly bound electrons are poor conductors (insulators).
Q: Does temperature affect electron flow?
A: Yes! Higher temperatures cause atoms to vibrate more, hindering the movement of electrons and increasing resistance. This is why electrical conductivity often decreases as temperature increases.